We published a paper entitled « Carbon polymorphism in shocked meteorites: Evidence for new natural ultrahard phases » in the recent issue of EPSL. This post is the uncorrected proof featuring all the references, tables and pictures we published in the paper. Comments are very welcome. If you want a reprint of the paper, please use thee Contact Form.
Carbon polymorphism in shocked meteorites : evidence for new natural ultrahard phases
Tristan Ferroir1, Leonid Dubrovinsky2, Ahmed El Goresy2, Alexandre Simionovici3, Tomoki Nakamura4 and Philippe Gillet1,
1 Laboratoire des Sciences de la Terre, Universite de Lyon, Ecole Normale Superieure de Lyon, Universite Claude Bernard Lyon 1, CNRS 46 Allee d’Italie, 69364 Lyon Cedex 07, France
2 Bayerisches Geoinstitut, Universitat Bayreuth, D-95440 Bayreuth, Germany
3 Laboratoire de Geodynamique des Chaînes Alpines, OSUG, BP 53X, 38041 Grenoble, France
4Department of Earth and Planetary Sciences, Kyushu University, Hakozaki, Fukuoka 812-8581, Japan
Abstract
The ureilite class of meteorites is named after the type example Novo-Urei, Russia that fell in 1886 and is one of the unusual achondritic meteorites. Ureilites contain olivine and pyroxenes (pigeonite, augite, or orthopyroxene but seem to be barren of feldspars) with graphite-bearing veins (including tiny diamonds), Fe metal with very low Ni-content, troilite, Fe3C and other minor accessory phases. A slice of the Havero ureilite was cut and then polished as a thin section using a diamond paste.. We identified two carbonaceous areas which were standing out by more than 10 /jm in relief over the surface of the silicate matrix suggesting that the carbonaceous phases were not easily polishable by a diamond paste and would therefore imply larger polishing hardness. These areas were investigated by reflected light microscopy, high-resolution Field Emission SEM (FESEM), energy-dispersive X-ray (EDX) analysis, Raman spectroscopy, and were subsequently extracted for in situ synchrotron microbeam X-ray fluorescence (XRF), imaging and X-ray diffraction (XRD). We report here the natural occurrences of one new ultrahard rhombohedral carbon polymorph and the theoretically predicted 21R diamond polytype in the Havero meteorite thus demonstrating that the carbon system is even more complex than what is currently thought based on ab initio molecular dynamic simulations and high-pressure experiments..
1. Introduction
Since the discovery of fullerenes[14] and carbon nanotubes[12], four forms of pure carbon are now recognized including diamond and graphite. These discoveries enhanced the interest in exploring possible further occurrences of polymorphs and polytypes of carbon. Many applications are foreseen for these new materials such as the ultrahard carbon polymorphs, possibly harder than diamond[9, 5, 22, 20]. A new and theoretically unpredicted ultrahard carbon polymorph formed under a very intense and brief shock was found in gneisses from the Popigai impact crater [6] and theoretically investigated afterwards [19]. This lead us to document the nature of shocked carbon materials in the ureilite class meteorites, particulary the Havero ureilite [24], which contains about 3% wt of pure carbon.
2. Materials and methods
A piece of the Havero meteorite was polished as a thin section using a diamond paste powder and was observed in reflected light microscopy. We identified several carbonaceous areas which were standing out by more than 10 /jm in relief compared to the silicate matrix. These areas were investigated by SEM, EDX and Raman spectroscopy and were afterwards extracted to be investigated by means of Synchrotron Radiation X-ray fluorescence and X-ray diffraction. We conducted SEM imaging on two of the three areas on an environmental SEM, FEI XL 30 FEG ESEM at GEMPPM, INSA Lyon, France. Standard SEM could not be used for that kind of sample since we needed to avoid carbon or gold thin film deposition. Raman experiments were performed at the Laboratoire des Sciences de la Terre (ENS Lyon, France). The spectra were recorded on a XY ©DILOR Raman microspectrometer equipped with a CCD detector. The spectrometer was used in backscattering geometry. The laser beam (514.5 nm exciting lines of a Spectra Physics@ Ar+ laser) was focused through microscope objectives (x100) down to a 1 micrometer spot on the sample and the backscattered light was collected through the same objective. To avoid any amorphization of the carbon phase, density filters were used so that the power on the sample was no more than 50 pW. Spectra were collected in the spectral region of 1200 to 1650 cm-1. On specific regions, larger spectra were acquired in the range of 200 to 3200 cm-1. The areas were subsequently drilled out of the thin section and placed at the center of a drilled stainless steel holder to allow X-ray diffraction and X-ray fluorescence on two different synchrotron beamlines at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. On the /x-FID beamline (micro fluorescence imaging and diffraction beamline, ID22), the sample was placed at 45° from the incident beam, facing an optical microscope in order to precisely locate the area of interest. A Si(Li) X-ray fluorescence detector was disposed at 90° from the incident beam while a CCD camera was placed 10 cm behind the sample to collect X-ray diffraction images. The beam was collimated through slits and focused by K-B mirrors down to a 4×1,5 /лm2 beamspot and a high flux of 1011 ph/s. We used a 22 keV energy beam to collect X-ray diffraction. Both fluorescence and diffraction were collected simultaneously with a dwell time of 20 s. A movable PIN diode could be placed after the sample to collect transmission flux allowing identification and characterization of the areas according to their mean density. Preliminary analysis consisted of a fine scale mapping of the different areas both in X-ray fluorescence and diffraction. Fluorescence mapping results were then carefully studied to select specific points to be investigated more precisely by X-ray diffraction. A second run of investigation was done on the High Pressure beamline (ID09) mainly dedicated to high pressure experiments and equipped only for X-ray diffraction. At this beamline, the beam is only collimated using slits down to a size of 10 by 10 /jm. The sample holder is mounted on a rotating device which allows both visual observation when at 90° position and X-ray diffraction when facing the beam. The energy is higher than at the /x-FID beamline (30keV) and the set-up is optimized for X-ray diffraction.
3. Results
We identified two carbonaceous areas standing out by more than 10 /jm in relief over the surface of the silicate matrix. This height suggests that the carbonaceous phases were not easily polishable by a diamond paste and would therefore imply higher polishing hardness. These high relief zones are surrounded by flat polished culets and powdery darker areas. The reflected light image (Fig. 1) shows that the polished surface of the high relief zone is irregularly scalloped with several gouges, granular, microtextured with a concentric spatial arrangement. The height varies within individual multiphase grains between 5 and 12 /jm. This is strongly suggestive of the presence of different coexisting transparent materials with different polishing hardness. Synchrotron XRF analysis showed that the area is composed of pure carbon and depleted in high Z elements (including Fe, Mn, Ni and Zn) in comparison with the surrounding silicate matrix. Fe- and Ni-bearing metal particles are sprinkled in the C-enclave and also occur in the underlying silicates. Four different carbon phases were identified by Raman spectroscopy (Fig. 2) : graphite, diamond (3C-polytype) and two so far unknown carbon phases. A similar spatial (concentric relationship between the different zones) and mineralogical arrangement of the carbonaceous phases is identified in the two carbonaceous areas. The low-relief lithology in the carbon-rich enclave (Figure 1, zone A) mainly consists of disordered graphite depicting small graphite D and D’ bands, a sharp G band spectrum[3, 4] and diamond projecting 2 /jm over it with its characteristic one-phonon band [15]. The high relief area (Figure 1, zone B) standing 8 /jm above the carbonaceous matrix exhibits a small Raman band of diamond which is shifted to 1322 cm-1 (characteristic of lonsdaleite or nanodiamonds) and a sharp G band indicative of ordered graphite. Finally, the very high relief zone (Figure 1, zone C) shows diamond and main graphite bands together with several additional peaks not attributable to any known carbon phase. The new phases of zone C are also mixed with diamond and ordered graphite. The concentric occurrence and the relationship between the different carbon polymorphs have to be interpreted in terms of phase transitions during the pressure and temperature variations throughout the shock event undergone by the meteorite. Synchrotron X-ray diffraction data recorded on the first carbonaceous area is consistent with the Raman data confirming the presence of graphite, diamond and a new phase (Fig 3 a, b). The analysis of the 2D diffraction images shows that diamond and graphite have strong
preferred orientations and that the graphite [00l] reflection is parallel to the diamond’s [111]. The X-ray pattern can be successfully indexedindicating pure iron, mainly coming from a lower level in the thin section, diamond 3C, secondary uncompressed graphite and a new diamond polytype, the 21R[27, 11, 28, 16]. Best fit for the 21R polytype was achieved for a R-3m space group and cell parameters of a=2.553(1)A and c=44.490(6) A for a volume of 251.19(4) A3 close to the theoretical predictions[25, 10, 23]. This polytype is observed for the first time in natural materials. In the second studied area of the sample, most of the diffraction patterns can be indexed as graphite, diamond and/or lonsdaleite. In the highest part of the carbonaceous inclusion the diffraction pattern confirmed the following species : compressed graphite (with lattice parameters a=2.454(1)A and c=6,372(1)A versus lattice parameters of secondary (or re-crystallised) graphite a=2.462(1)A and c=6.704(1)A), lonsdaleite and/or diamond (3C polytype), and a new phase. The new phase has a distinctly new diffraction pattern also different from the 21R polytype, thus confirming the occurrence of a new carbon polymorph. The unit cell contains four structural positions C1 (0.276, 0.276, 0.276), C2 (0.273, -0.256, -0.256), C3 (0., 0., 0.), C4 (0.5, 0.5, 0.). Although the quality of the available diffraction data is not sufficient for a full-profile structural refinement, processing of the diffraction pattern with fixed structural positions and optimisation occupancy factors indicates that the C3 and C4 positions are just partially filled which makes the density of the new phase intermediate between the densities of graphite and diamond. Figs. 3c, 3d show the diffraction patterns collected from a small area of the grain (5×7 /лm2) with a very high relief. In addition, to the diffraction lines of graphite and diamond (or lonsdaleite) the pattern reveals additional lines. While the lines at 2.055 ˚ 1.257Д 1.450Д and 1.185A are close to the lines of lonsdaleite and/or diamond, the other lines cannot be assigned to diamond or its polytype. The presence of the common texturing features on all intense diffraction lines (Fig. 3c) indicates that all of them belong to the same phase. The X-ray pattern was successfully indexed in terms of the Pm3m space group. Indeed, all lines indexed in the framework of a cubic primitive lattice with the cell parameter 3.559(4)A for a cell volume V = 45.08A ˚ . However, some reflections are asymmetric (particularly, the (111) reflection shown in the inset in Fig. 3d), while others (for example, (200)) are not. This means the actual symmetry of the new phase is lower than cubic and the best fit was obtained for a rhombohedral lattice (R3m space group) with the parameters a=3.5610(9) ˚ a=90.2(2)°. The proposed crystal structure of the new carbon polymorph is closely related to the structure of diamond : all carbon atoms are tetrahedrally coordinated, but slightly shifted from their symmetric positions in the diamond structure and two of the four structural positions are partially occupied. The presence of weak satellite reflections at 2.182A and 1.928A (if not coming from lonsdaleite) may also indicate a significant degree of disorder of the crystal structure.
4. Discussion
The observation of both compressed and uncompressed graphites and diamond show that a complex history and relationship exists between the different carbon phases. The existence of the compressed graphite strongly advocates for a shock event, which would entail the compression of pre-existing graphite. The dispersion of the FWHM and positions of the diamond Raman band [15] in different spots of the carbonaceous inclusions also strongly suggests a shock event. Using the average positions of the main Raman band of graphite and the value of its shift with pressure[21], we can calculate a residual stress of about 2.5 GPa. Similar stresses ranging from 2.5 to 4 GPa are inferred from the X-ray diffraction data using the equation of state of graphite[8]. A theoretical model of the diamond to graphite conversion[26] has shown that the topotaxial relationship between diamond (111) and graphite (002) could explain the formation of graphite islands inside the CVD diamonds[29] at temperatures higher than 2000 K. However, observations of graphitic islands inside CVD diamonds can be made for lower temperatures. More recently, Guillou et al. [7] showed that by static compression of black carbon at high-pressure and high-temperature, it is possible to obtain such a topotaxial relationship between diamond and graphite. The obtained product also exhibits very complex Raman spectra which are not observed when polycrystalline graphite or highly oriented pyrolitic graphite (HOPG) is used as a starting material. Even though, their Raman results do not exactly match the same bands as our samples, they show that the nature and P-T history of the diamond precursor material has a strong influence on the formation of diamond and other related materials. Therefore, a shock event on a graphitic precursor material ( whose residues are now present as primary compressed graphite) would have allowed to partially transform graphite to diamond 21R polytype and a new high-pressure phase of carbon. A high post-shock temperature entailed a partial back-transformation of diamond to the secondary uncompressed graphite observed in the sample. Carbon phases with “diamond-like” diffraction patterns but in space group Fd3m and forbidden reflections have already been described as products of shock-wave, ion implantation experiments with graphite or chemical treatments of diamond[9, 18, 13, 2]. The possible existence of a diamond-like structure with only partially occupied atomic positions (within the Fm3m space group) was also already discussed[17]. However, we present for the first time a structural model based on powder diffraction data of a natural carbon polymorph most likely intermediate between graphite and diamond. Note that the existence of such a phase has not hitherto been theoretically predicted[20, 22, 17]. The high polishing hardness of the new polymorph and the 21R polytype could be explained by the preferred orientations confirmed from the x-ray diffraction patterns and the heterogeneous hardness along the different crystallographic planes : highest hardness is at the (111) plane in analogy with 3C polytype. These observations of diamond 21R polytype and the new rhombohedral carbon polymorph show that the carbon system is far more complex than previously thought. The main information arising from these discoveries is that shocked carbon opens a new vision of the carbon system. Notwithstanding, the conditions to reproduce natural shock conditions cannot be reached experimentally since only nano- to microseconds shock durations can be achieved, while for natural objects, due to the significant size of the impactors, durations of shock events could be several seconds long[1]. Therefore, theoretical calculations have to show whether shock simulations can predict these new phases. The new carbon phases found in the Havero ureilite are thought to be metastable at ambient conditions and indeed formed at high-pressure in a purely dynamic event. They could represent intermediate states in the transformation of graphite to diamond. We maintain that the study of naturally shocked samples can greatly improve our knowledge of the carbon system since it gives access to unreachable areas of the phase space.
Acknowledgements The authors thank Gilles Montagnac for technical assistance with Raman spec-troscopy. We also thank Remi Tucoulou (ID22, ESRF) and Michael Hanfland (ID09, ESRF) for help during X-ray diffraction experiments. MATEIS Lab and A. Bogner is also thanked for providing access to the SEM facility and operating the environmental SEM. This project was supported by the ANR project ECSS.
References
[1] P. Beck, P. Gillet, A. El Goresy and S. Mostefaoui. Timescales of shock processes in chondritic and martian meteorites. NATURE, 435(7045) :1071-1074, June 2005. ISSN 0028-0836.
[2] Natalia Dubrovinskaia, Leonid Dubrovinsky, Falko Langenhorst, Steven Jacobsen, and Christian Liebske. Nanocrystalline diamond synthesized from c60. Diamond and Related Materials, 14(1) :16-22, 2005. doi : 10.1016/j.diamond.2004.06.017.
[3] A. C. Ferrari and J. Robertson. Interpretation of raman spectra of disordered and amorphous carbon. Physical Review B, 61 :1313119-14107, May 2000.
[4] A. C. Ferrari and J. Robertson. Resonant raman spectroscopy of disordered, amorphous, and diamondlike carbon. Physical Review B, 64 :75414, August 2001.
[5] Yury G. Gogotsi, Andreas Kailer, and Klaus G. Nickel. Pressure-induced phase transformations in diamond. Journal of Applied Physics, 84 :1299-1304, 1998.
[6] Ahmed El Goresy, Leonid S. Dubrovinsky, Philippe Gillet, Smail Mostefaoui, Gunther Graup, Michael Drakopoulos, Alexandre S. Simionovici,
Varghese Swamy, and Victor L. Masaitis. A new natural, super-hard, transparent polymorph of carbon from the popigai impact crater, russia. Comptes Rendus Geo-sciences, 335(12) :889-898, October 2003. doi : 10.1016/j.crte.2003.07.001.
[7] Corentin Le Guillou, Fabrice Brunet, Tetsuo Irifune, Hiroaki Ohfuji, and Jean-Noel Rouzaud. Nanodia-mond nucleation below 2273 k at 15 GPa from carbons with different structural organizations. Carbon, 45(3) :636-648, March 2007. doi : 10.1016/j.carbon.2006.10.005.
[8] M. Hanfland, H. Beister, and K. Syassen. Graphite under pressure : Equation of state and first-order raman modes. Physical Review B, 39(17) :12598, 1989. Copyright (C) 2008 The American Physical Society; Please report any problems to prola@aps.org.
[9] Hisako Hirai and Ken-Ichi Kondo. Modified phases of diamond formed under shock compression and rapid quenching. Science, 253 :772-774, August 1991.
[10] C. E. Holcombe. Calculated x-ray diffraction data for polymorphic forms of carbon. Report No. Y-1887, Oak Ridge Y-12 Plant, Oak Ridge, TN, 1973.
[11] T. Hom, W. Kiszenik, and B. Post. Accurate lattice constants from multiple reflection measurements. II. lattice constants of germanium silicon, and diamond. Journal of Applied Crystallography, 8(4) :457-458, 1975.
[12] Sumio Iijima and Toshinari Ichihashi. Single-shell carbon nanotubes of 1-nm diameter. Nature, 363 : 603-605, June 1993.
[13] I. Konyashin, A. Zern, J. Mayer, F. Aldinger, V. Babaev, V. Khvostov, and M. Guseva. A new carbon modification ’n-diamond’or face-centred cubic carbon? Diamond & Related Materials, 10(1) :99-102, 2001.
[14] H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl, and R. E. Smalley. C60 buckminsterfullerene. Nature, 318(6042) :162-163, November 1985. doi : 10.1038/318162a0.
[15] M. Miyamoto, T. Takase, and Y. Mitsuda. Raman spectra of various diamonds. Mineralogical Journal, 16(5) :246-257, 1993.
[16] P. D Ownby, X. Yang, and J. Liu. Calculated x-ray diffraction data for diamond polytypes. Journal of the American Ceramic Society, 75(7) :1876–1883, 1992.
[17] C. J Pickard, V. Milman, and B. Winkler. Is there theoretical evidence for a metallic carbon polymorph with space group symmetry fm3m at ambient conditions? Diamond & Related Materials, 10(12) :2225– 2227, 2001.
[18] S. Prawer, J. L. Peng, J. O. Orwa, J. C. McCallum, D. N. Jamieson, and L. A. Bursill. Size dependence of structural stability in nanocrystalline diamond. Physical Review B, 62(24) :16360–16363, 2000.
[19] Filipe J. Ribeiro, Paul Tangney, Steven G. Louie, and Marvin L. Cohen. Hypothetical hard structures of carbon with cubic symmetry. Physical Review B (Condensed Matter and Materials Physics), 74(17) : 172101–4, November 2006.
[20] S. Scandolo, G. L. Chiarotti, and E. Tosatti. SC4 a metallic phase of carbon at terapascal pressures. Physical Review B, 53 :5051–5054, March 1996.
[21] T. L. Schindler and Y. K. Vohra. A micro-Raman investigation of high-pressure quenched graphite. Journal of Physics Condensed Matter, 7 :L637–L642, November 1995.
[22] S. Serra, G. Benedek, M. Facchinetti, and L. Miglio. Possible high-pressure phase of diamond. Physical Review B, 57 :5661–5667, March 1998.
[23] K. E Spear, A. W Phelps, and W. B White. Diamond polytypes and their vibrational spectra. Journal of Materials Research, 5(11) :2277–85, 1990.
[24] G. P. Vdovykin. Forms of carbon in the new havero ureilite of finland. Meteoritics, 7 :547, 1972.
[25] A. R Verma and P. Krishna. Polytypism and polymorphism in crystals. New York, 1966.
[26] Alessandro De Vita, Giulia Galli, Andrew Canning, and Roberto Car. A microscopic model for surface-induced diamond-to-graphite transitions. Nature, 379(6565) :523–526, 1996. doi : 10.1038/379523a0.
[27] D. R. Wilburn and W. A. Bassett. Hydrostatic compression of iron and related compounds; an overview. American Mineralogist, 63(5-6) :591–596, 1978.
[28] R. W.G Wyckoff. Crystal structures. Wiley, New York, 1963.
[29] W. Zhu, C. A. Randall, A. R. Badzian, and R. Messier. Graphite formation in diamond film deposition. Journal of Vacuum Science & Technology A : Vacuum, Surfaces, and Films, 7(3) :2315–2324, 1989.
N dobs., A h к 1 dcalc, A I, % Phase
| 1 | 3,276 | 002 | 2,9 | Gr | |
| 2 | 2,182 | 1 00 | 3,1 | L | |
| 3 | 2,055 | 1 1 1 | 2,055 | 100 | N* |
| 4 | 1,928 | 1 0 1 | 2,3 | L | |
| 5 | 1,780 | 200 | 1,780 | 7,2 | N |
| 6 | 1,451 | 2 1 1 | 1,543 | 5,1 | N |
| 7 | 1,257 | 2 2 0 | 1,258 | 3,7 | N |
| 8 | 1,185 | 3 0 0 | 1,186 | 14,2 | N |
| 9 | 1,072 | 3 1 1 | 1,073 | 10,8 | N |
| 10 | 1,024 | 2 2 2 | 1,027 | 2,7 | N |
Tab. 1: The X-ray pattern was successfully indexed in terms of the Pm3m space group. Indeed, all lines easily indexed in the framework of rhombohedral symmetry and lattice parameters a = 3.5610(9)A, a = 90.2(2)°. It was then successfully refined using the LeBail algorithm found in the GSAS package and the derived structural model was obtained from the Endeavour program.
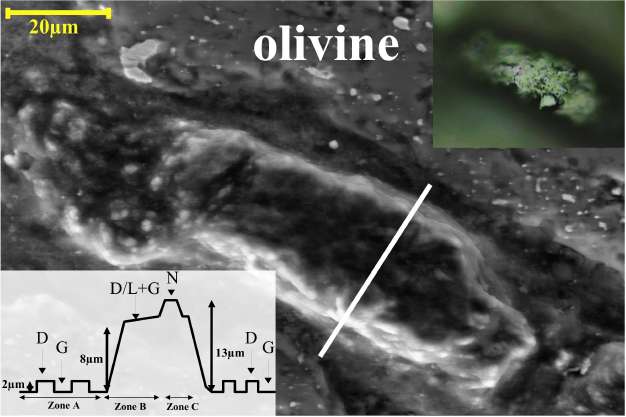
Fig. 1: One of the carbonaceous areas in the Havero ureilite. The optical microscopy image (top right) and SEM image (center) of a typical carbonaceous area in Havero (area containing the 21R polytype). The SEM image shows the different heights in the carbonaceous area. The lower left inset shows a scheme of the spatial concentric arrangement of the different carbonaceous areas through a section represented by the solid white line on the SEM picture.
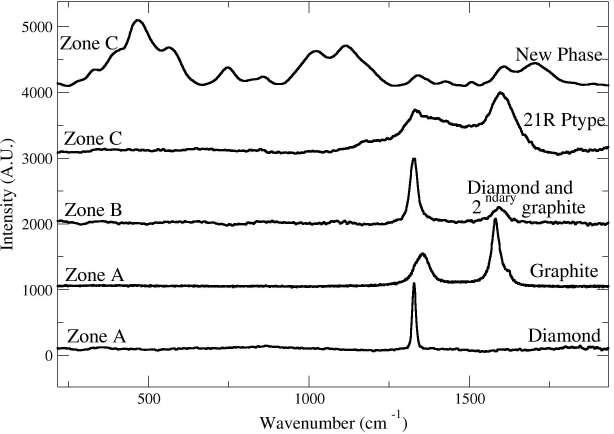
Fig. 2: Raman spectra obtained from the different carbon phases in the Haver¨o ureilite. (The labeling of the spectra corresponds to the areas indicated in Figure 1). Diamond with its typical one phonon band at 1331 cm-1 together with either secondary graphite and its characteristic G band at 1582 cm-1 or disordered graphite with D and G band at 1375 and 1572 cm-1 are observed. The two new phases exhibit bands belonging to diamond and graphite but also additional bands which are located at 442, 538, 1010, 1177, 1214, 1412, 1495 cm-1 for the 21R-polytype and some Raman bands at 336, 380, 468, 567, 750, 863, 1027, 1122, 1211, 1419, 1508, 1604 and 1700 cm-1 for the new carbon phase. Although some bands of the precited phases are usually attributed to classical fundamental and defect modes of graphite (1080, 1200, 1350 and 1500 cm-1), to lonsdaleite (1280 cm-1) or to domain size effects (580 cm-1), most of these bands have never been observed in any known carbon species.

Fig. 3: 2D (left) and integrated (right) diffraction patterns recorded in the highest relief zones of the two studied carbonaceous area. (a) The image of area 1 (Fig 1) at a wavelength of 0.412A (ID09, ESRF) shows a simultaneous directional increase of the intensities of the graphite (002) and diamond (111) lines suggesting a topotaxial preferred orientation relationship between the two phases. (b) The integrated pattern was successfully indexed as a mixture of graphite (G), diamond (D), 21R polytype of diamond (21R) and bcc-structured iron-nickel alloy (I), and further refined using the LeBail algorithm as implemented in the GSAS package. * stands for peaks originating from the lower silicate matrix. Blow-up inset figure at the top right shows the area around the diamond (111) peak where most of the 21R diffracting planes can be seen and identified unambiguously.(c) The image of area 2 at a wavelength of 0.6199 A (ID22, ESRF) shows diffraction lines attributed to the new super hard carbon polymorph (marked by “N” and indexed in pseudo-cubical settings), graphite (G), and reflection rings from lonsdaleite (L). The integrated diffraction pattern is interpreted as a mixture of diamond (3C polytype)(D), small amounts of compressed graphite and a new carbon phase (N) with rhombohedral symmetry and lattice parameters a = 3.5610(9)A, a = 90.2(2)°. A close-up view shows the asymmetry of the N(111) peak suggesting a splitting into N(111) and N(11-1) advocating for the rhombohedral symmetry.

I read your article in Earth and Planetary Science Letters (Feb 15th 2010) with interest since I work in the area of CVD diamond deposition and characterisation. A few years ago I published a paper in DRM concerning analysis of nanocrystalline CVD diamond films using 785 nm Raman, and found a complex spectrum consisting of dozens of ‘new’ peaks. The evidence seemed to suggest we were probing the interface/nucleation layer between the diamond film & the Si substrate, where the diamond grain size was of the order of a few 10’s of nm.
We were never able to identify most of these peaks, and vaguely assigned them to a combination of carbon polymorphs or defective diamond.
Interestingly, when I saw the list of peak positions in your two new polymorphs of carbon/diamond from the meteorite, many of the peak positions corresponded quite closely with the ones we saw in the CVD film (see Table 1 in our paper). So I wonder if the meteorite diamond polymorphs and those we saw at the nucleation layer of a CVD diamond film are the same, or closely related, materials?
best wishes,
Thank you very much for your comments. Your paper is really interesting because of the Raman spectra you present. We indeed think that the grain size of our phase is very small because our Raman spectra are very different from one point to another even if we move than less than a micron. However, as you read in the paper, we used a 514nm laser. If we compare to your spectra at the same wavelength, our is totally different which would mean that the bonding of our phase compared to the bonding of your CVD material is different.
It is however possible that we have related materials but it is nearly impossible that our phase has been formed by vapor deposition. We think that it is formed by shock. However, some bonding between the interface of nucleation and our phase could be the same since our nucleation sites are either diamond/graphite or silicate materials.